Team
Toxoplasmosis & Host-parasite coevolution
Dpt: Environnement, Reproduction, Infections, Cancer
Our research activities
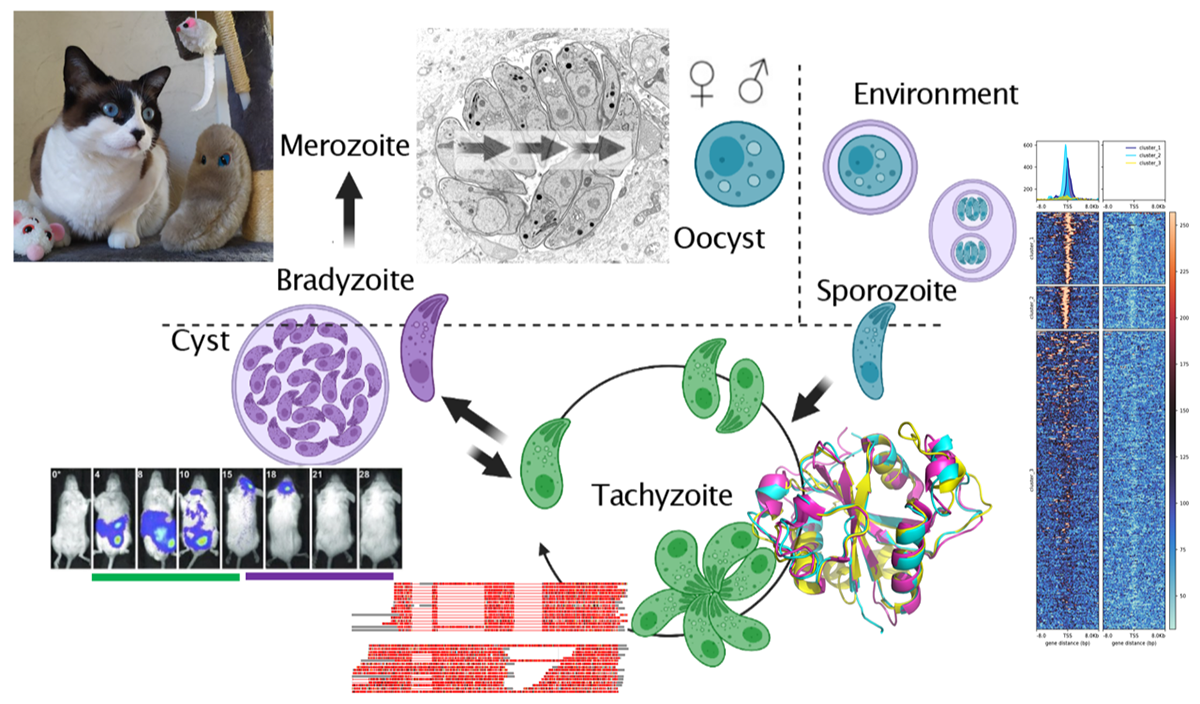
The parasite Toxoplasma gondii is the etiologic agent of toxoplasmosis, a worldwide foodborne zoonosis that is particularly severe when opportunistic or congenital. As an obligate intracellular parasite, Toxoplasma unfolds sophisticated mechanisms to profoundly remodel its host cell niche. Our team is investigating how the parasite is able to hijack host cell signaling pathways after invasion. In doing so, we have discovered a new subfamily of effector proteins that are exported by the parasite into the host cell nucleus to reshape the host's epigenetic program. Our goal is to investigate the modus operandi of these effectors and, in particular, their potential impact on immune evasion, parasite persistence, and transmission to new hosts. A hallmark of the Toxoplasma life cycle is the multiplicity of developmental stages, with the transition from one stage to the next involving precise genetic reprogramming to ensure survival and transmission of the parasite population. We have assigned a role to chromatin shapers, including histone modifications, in determining specific epigenetic programs for each stage. We are studying the function of these molecular epigenetic switches with the goal of manipulating them to induce sexual development in vitro. Our research is also relevant to applications as we have developed serological markers for chronic toxoplasmosis and novel therapeutics to cure toxoplasmosis and other parasite-borne diseases such as malaria.
Our research axes
A hallmark of Toxoplasma infection is that the parasite actively diverts host signaling pathways from their original functions to promote its persistence and transmission to multiple hosts. We are studying how the parasite epigenetically alters host gene expression by injecting effector proteins into the cells it invades. This arms race between the parasite and its hosts has led to accelerated adaptive evolution of effector proteins that counteract and limit the strength of the immune response.
Learn moreToxoplasma has a complex life cycle with multiple stages and hosts. All developmental stages have their own transcriptional signature, and switching between stages is controlled by intricate transcriptional cascades in which covalent and non-covalent epigenetic mechanisms act as driving forces. We are investigating how parasite chromatin modifiers cooperate with specific transcription factors to epigenetically regulate the transition to chronicity and sexual development.
Learn moreApicomplexa are responsible for major human infectious diseases such as toxoplasmosis, cryptosporidiosis and malaria. For many of these diseases, current treatments are suboptimal and few alternatives exist. We are exploring the chemical diversity of off-patent drug libraries to identify new pan-apicomplexan drug candidates. Our long-term goal is to feed the antiparasitic drug pipelines with novel drug scaffolds linked to an alternative mode of action to move into preclinical development.
Learn moreSerologic testing has long been the first-line means of confirming Toxoplasma infection, but current serologic diagnosis does not always distinguish between acute, latent, and reactivated disease states. Reliable specific serological markers for tissue cysts/bradyzoites have been lacking for both research and medical diagnosis. By developing a new diagnostic toolkit to detect persistent parasites in patient sera, we hope to improve the diagnosis of chronic toxoplasmosis in human medicine.
Learn moreOur major publications
See all publicationsOur activities in pictures
Our collaborations
- Matthew Bowler (EMBL-Grenoble outstation, France)
- Amit Sharma (ICGEB, New Delhi, India)
- Jeroen Saeij (UC Davis, USA)
- Isabelle Coppens (Johns Hopkins University, Baltimore, USA)
- L. David Sibley (Washington University School of Medicine, St. Louis, USA)
- Dominique Soldati-Favre (Geneva university, Switzerland)
- Antonio Barragan (Stockholm University, Stockholm, Sweden)
- Fabrice Laurent (INRAe, Tours, France)
- Artur Scherf (Pasteur Institute, Paris, France)
- Thierry Lagrange (CNRS, Perpignan, France)
- Isabelle Tardieux (CNRS, Grenoble, France)
Our technologies
- Cell culture and gene editing (CRISPR-Cas9) in Toxoplasma gondii
- Cell biology (epifluorescence, confocal)
- Gene expression analyses (Illumina, Nanopore DRS, qRT-PCR)
- Epigenetic analysis (ChIP-seq, ATAC-seq)
- Conventional and affinity biochemistry
- Recombinant protein expression (E. coli, Insect cell)
- Structural biology (Crystallization, TSA, ITC, Microscale thermophoresis, Alpha Fold)
- Immunology (ELISA, FACS)




