Our research axes
Toxoplasmosis & Host-parasite coevolution
Investigate how effectors secreted by Toxoplasma contribute to immune evasion and persistent parasitism
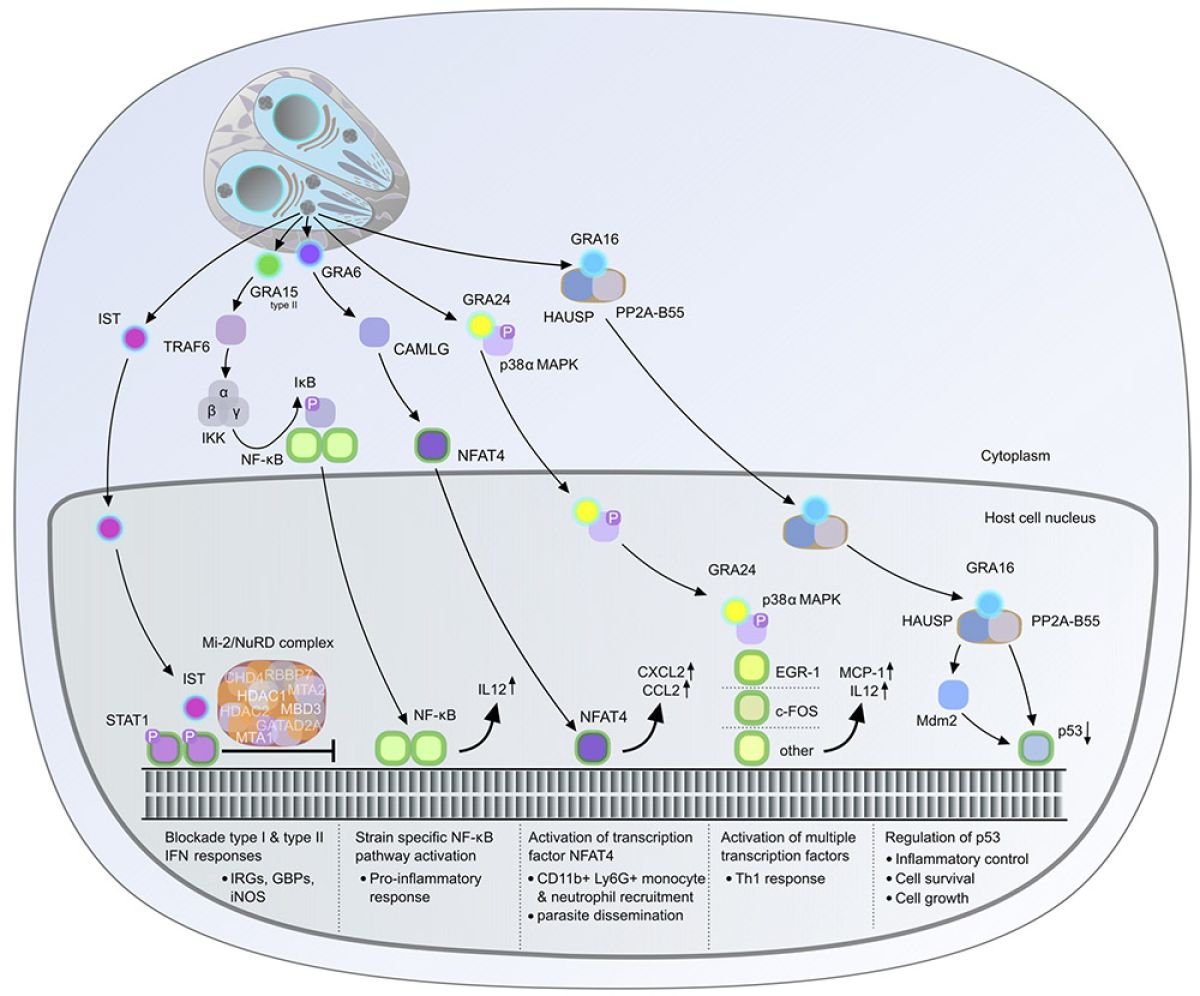
A hallmark of Toxoplasma infection is that the parasite actively diverts host signaling pathways from their original functions to promote its persistence and transmission to multiple hosts. Our research focuses on how the parasite alters the expression of host genes through epigenetic modifications facilitated by the injection of effector proteins into host cells. The past decade has been an exciting time for Toxoplasma research, particularly for those interested in the interaction between the pathogen and its host cell. We now know that Toxoplasma introduces multiple rhoptry and dense granule proteins into host cells, which perform crucial roles in the host-pathogen interaction. This ongoing arms race between the parasite and its host has resulted in the accelerated evolution of effector proteins that counteract and limit the immune response (Hakimi et al. Clin Microbiol Rev. 2017; Hakimi, Annual Review of Microbiology, 2022).
Despite the limited number of characterized GRA effectors, it is possible to draw some general conclusions about their modus operandi (Fig. 1). These effectors may employ at least three alternative strategies to manipulate host gene expression, which are not necessarily mutually exclusive: (i) modulation of upstream signaling pathways, (ii) direct targeting of host transcription factor protein levels/activity, and/or (iii) alteration of histone packing and chromatin configuration. Although the functions of some GRA proteins have been elucidated, the role of others remains unknown. Our research team is building on our previous findings and aims to:
- Identify the complete repertoire of GRA effectors and quantify the extent of their impact on the infected cell
- Explore the synergistic and/or antagonist effects of GRA effectors on gene regulation.
- Determine their contribution to immune evasion and sustained parasitism.
- Gain a deeper understanding of the function of GRA effectors by studying their three-dimensional structure when interacting with host cell factors. This will guide further experiments to investigate their function in more detail.
Explore the transcriptional switches that control Toxoplasma dormancy and sexual commitment
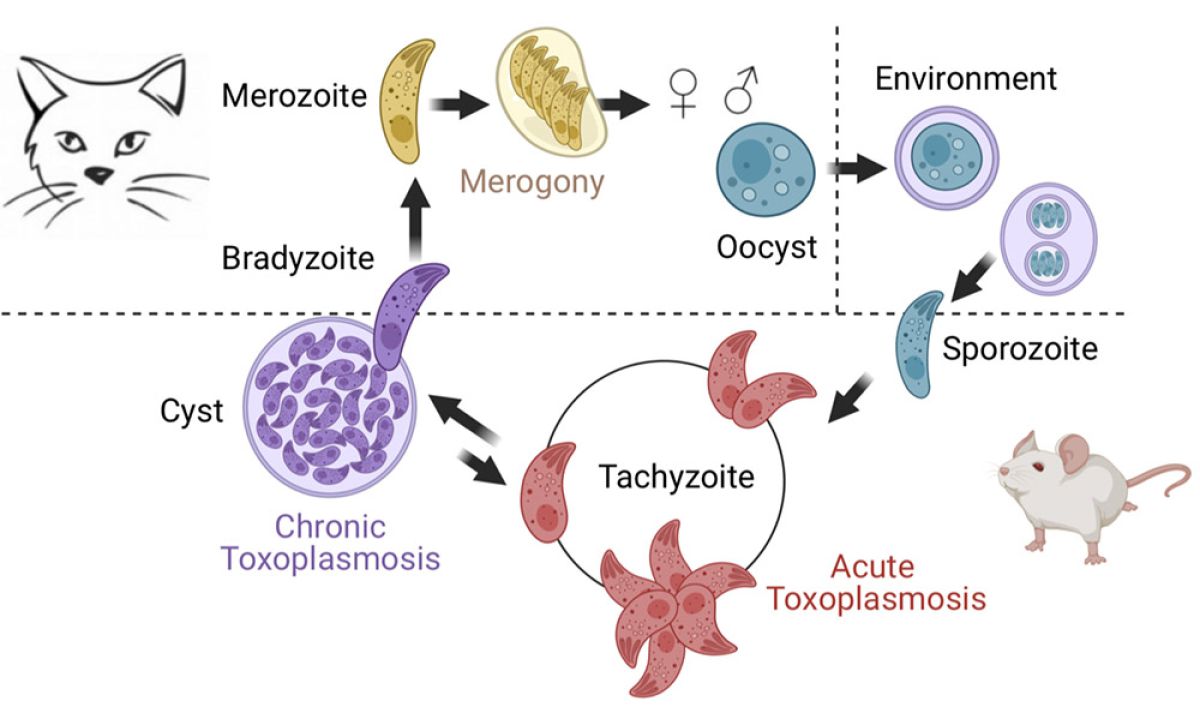
Toxoplasma has a complex life cycle with multiple stages and hosts (Fig. 2). All developmental stages have their own transcriptional signature, and switching between stages is controlled by intricate transcriptional cascades in which covalent and non-covalent epigenetic mechanisms act as driving forces. We are investigating how parasite chromatin modifiers cooperate with specific transcription factors to epigenetically regulate the transition to chronicity and sexual development.
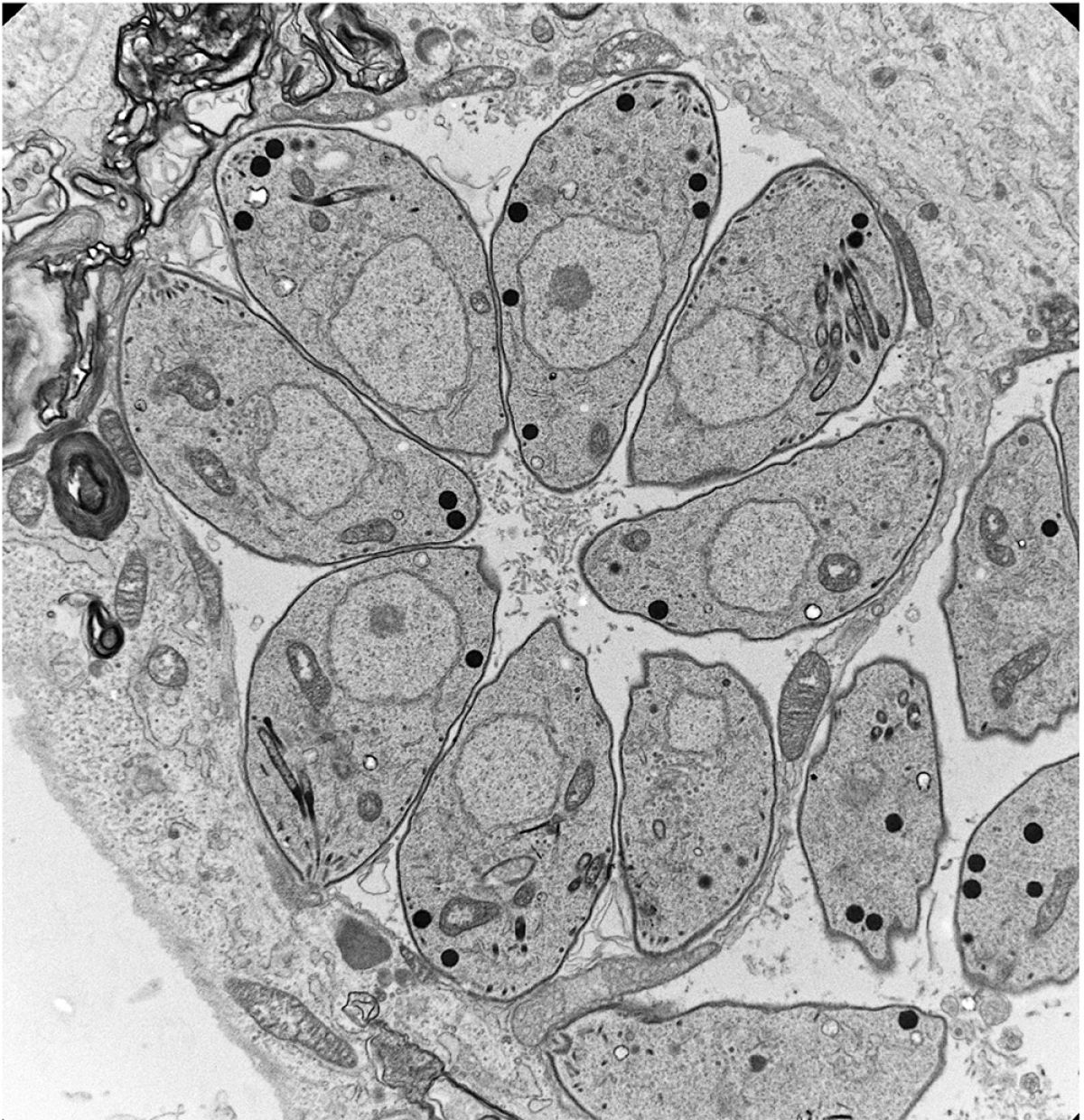
The challenge we faced was to reprogram the sexual forms of Toxoplasma by modifying the cell fate of its cultivable form, the tachyzoite. We developed and confirmed a model in which several epigenetic determinants govern the developmental trajectories of the parasite, as well as the unidirectionality of its heteroxenous life cycle. We identified a bimodal distribution of histone marks at the poised promoters of specific genes involved in the sexual stages within the tachyzoite. We then identified MORC, an unconventional chromatin remodeler that associates with the histone deacetylase HDAC3 to repress the expression of genes involved in the sexual stages. By genetically inactivating these regulators (MORC protein depletion) or using epigenetic drugs (HDACi), we demonstrated the major contribution of these regulators to parasite differentiation. More recently, we identified two transcription factors, AP2XII-1 and AP2XI-2, which cooperate with MORC/HDAC3 to control merogony, a key step in the sexual reproduction process observed only in the cat's intestine until now. By precisely manipulating these two genetic switches, we produced pre-sexual stages in vitro, studied their transcriptome and proteome, and confirmed the results of observations made in the 1970s by transmission electron microscopy. We also discovered new subcellular features and described in detail the unique process of endopolygeny by which these pre-gametes divide.
Our work provides an ethical alternative to the study of Toxoplasma's sexual reproduction, avoiding the infection of cats. Furthermore, our in vitro culture method represents a major advance in the fight against zoonosis by opening the way to the development of innovative therapies to block the transmission of the parasite from felines to their intermediate hosts, including humans. We are currently testing recently discovered drug candidates against the pre-gametes produced in vitro.
Repurpose drugs to cure neglected diseases transmitted by apicomplexan parasites
Toxoplasmosis, cryptosporidiosis, and malaria are major infectious diseases caused by Apicomplexa, for which current treatments are inadequate and alternative options are scarce. To address this, we are exploring the chemical diversity of off-patent drug libraries to discover new pan-apicomplexan drug candidates. Our aim is to provide novel drug scaffolds with an alternative mode of action for the antiparasitic drug pipeline and move into preclinical development. By conducting a screening of more than 4000 FDA-approved drug candidates, we have identified new compounds with nanomolar range inhibition of the growth of Toxoplasma, Cryptosporidium, and Plasmodium species, and high selectivity indexes. We plan to investigate the molecular targets of the best candidates through chemogenomics, validate them with CRISPR/Cas9-based genome editing using Toxoplasma (Fig. 3), and understand their mode of action at an atomic level by determining the crystallographic structure of molecules in complex with their target proteins. Finally, we will validate the lead compounds in animal models of Toxoplasmosis and Cryptosporidiosis to demonstrate their potential for future clinical use, while also enhancing our understanding of resistance mechanisms that may arise from widespread drug use in human and veterinary cases.
Development of a serological assay to detect dormant stage antigens and predict cyst portage in infected hosts
Chronic diseases have become a significant and constantly growing public health concern globally, leading to a rise in long-term treatment costs and disability-adjusted life years (DALYs) on global health measures. This issue includes infectious agents that are phylogenetically unrelated, such as viruses, bacteria, helminths, and protozoan parasites. Among these, Toxoplasma gondii, a protozoan parasite, is one of the most widespread microorganisms on Earth, capable of establishing long-term parasitism in all warm-blooded metazoans, including humans. In fact, around one-third of the world's population is presumed infected with Toxoplasma. The worldwide seroprevalence varies significantly by region due to various factors, such as climate, diet, hygiene, and host susceptibility. While toxoplasmosis usually presents itself in benign forms in individuals with healthy immune systems, it can cause severe and life-threatening symptoms in congenital infections and immunocompromised patients, particularly those with AIDS or transplants. Therefore, early and accurate diagnosis using sensitive and specific diagnostic tests is crucial in the prevention and treatment of severe toxoplasmosis.
Serologic testing has long been the first-line means of confirming Toxoplasma infection, but current serologic diagnosis does not always distinguish between acute, latent, and reactivated disease states. Reliable specific serological markers for tissue cysts/bradyzoites have been lacking for both research and medical diagnosis. Fortunately, a breakthrough was made when BCLA, a biomarker found to be restricted to the bradyzoite stage, was discovered by our team (Dard et al. BMC Biology, 2020). It was found to induce a strong humoral response in chronically infected mice and human patients with different forms of toxoplasmosis. Our aim is to utilize this biomarker to monitor the levels of circulating anti-BCLA antibodies in sera from i) patients undergoing serological reactivation (such as cancer therapies and post-transplant protocols), ii) those with confirmed ocular toxoplasmosis, and iii) a cohort of individuals with congenital Toxoplasma infections. We also intend to employ the BCLA-based marker to refine the epidemiological association between chronic infection and neuropsychiatric symptoms, particularly in individuals with schizophrenia and bipolar disorder. By developing a new diagnostic toolkit to detect persistent parasites in patient sera, we hope to improve the diagnosis of chronic toxoplasmosis in human medicine.